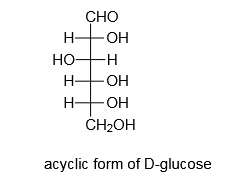
Ruff degradation is a reaction used to shorten the open chain forms of monosaccharides. It is functionally the reverse reaction of Kiliani-Fischer synthesis.
In 1898, Otto Ruff published his work on the transformation of D-Glucose to D-Arabinose later called the Ruff degradation. In this reaction, D-Glucose is converted to D-Arabinose. In this reaction, the terminal aldehyde group is converted to a carboxylic acid group, using selective oxidation of the aldehyde using Bromine water and then converted to gluconate ion. Next, Fe(OAc)3 with 30% of H2O2 is added.
Thus COO- ion will form CO2 and a stereo selective compound will form. And below -CH2OH will convert to -CHO group through the reduction of iron from its 3 state to 2 state, thus forming D-Arabinose.
This reaction was an important tool used by Emil Fischer to show that D-Glucose and D-Mannose each formed the same product upon Ruff degradation (D-Arabinose) indicating them to have opposite configurations at C-2 (epimers). Further Ruff degradation on D-Arabinose gave D-Glyceraldehyde, which established the stereochemistry of the chiral center on C-5.
See also
- Kiliani-Fischer synthesis
References